-
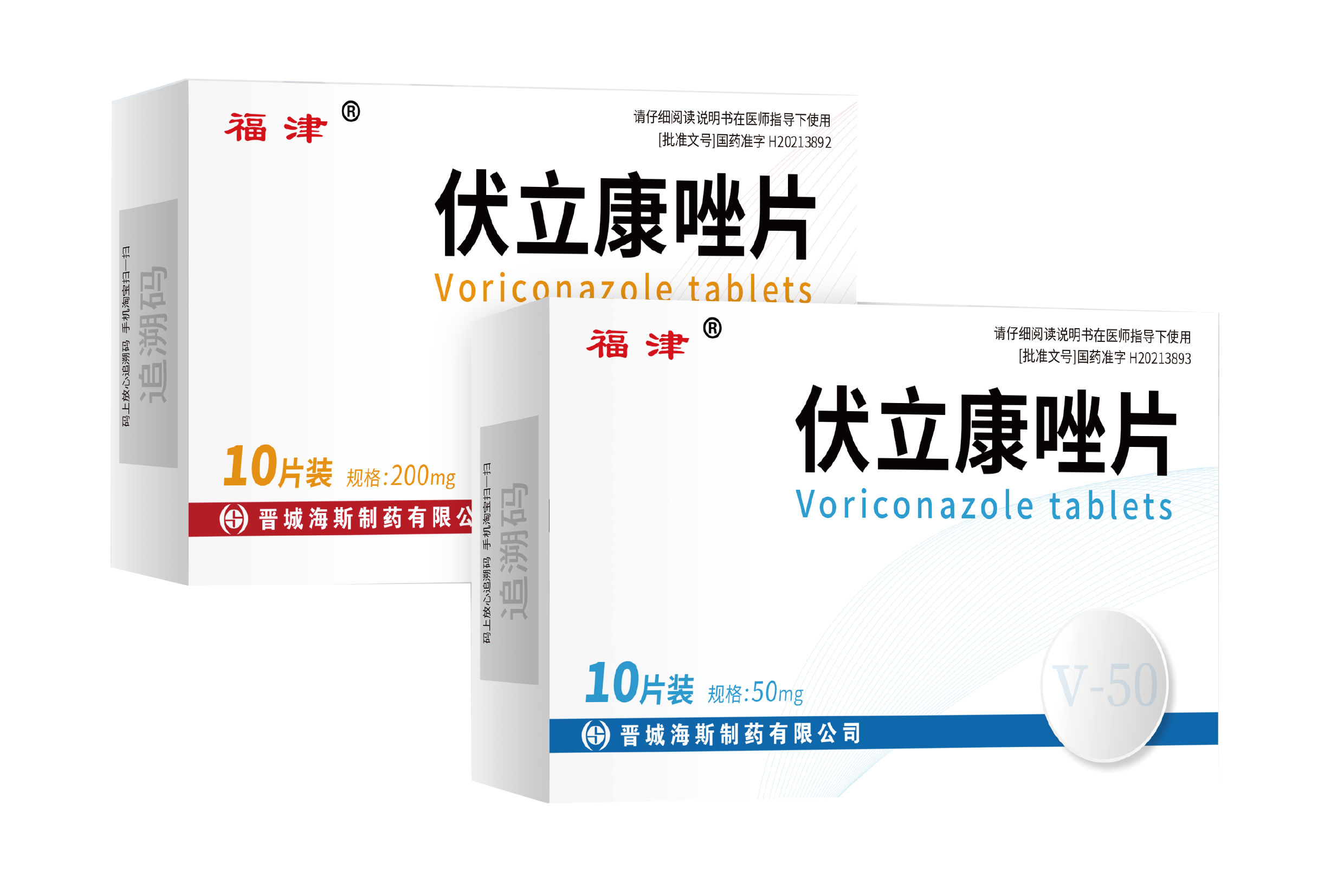 182025-06
182025-06Voriconazole tablets has been approved
Our new formulation Voriconazole tablets (50mg and 200mg) has been approved by China SFDA on 24th No -
 182025-06
182025-06Olopatadine hydrochloride tablets has been approved
Our new formulation Olopatadine hydrochloride tablets (5mg ) has been approved by China SFDA on 31st -
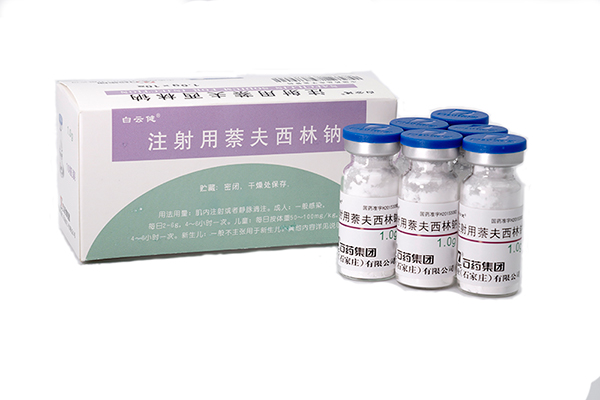 182025-06
182025-06Nafcillin Sodium for injection has been approved
Our new formulation Nafcillin Sodium for injection (1.0gram per ampoule) has been approved by China -
 182025-06
182025-06L-Ornithine-L-Aspartate Infusion for injection has been approved
Our new formulation L-Ornithine-L-Aspartate Infusion for injection( 5gm/10ml ) has been approved by -
 082024-05
082024-05Luliconazole and posaconazole are launched into market
The synthesis pilots of Luliconazole and posaconazole are successfully completed.The key intermediates are available to international market from July 2013.

 简体中文
简体中文
 86-10-67887855
86-10-67887855 Head Office:
Head Office: Marketing:
Marketing: Wista Pharmam
Wista Pharmam