Olopatadine hydrochloride tablets has been approved
Release time:2021-12-12


Our new formulation Olopatadine hydrochloride tablets (5mg ) has been approved by China SFDA on 31st May 2022. The tablets registration certificate No. is H20213892 and H20223350.
Reprint statement: reprint please indicate "source: Wista pharma".
Back
Voriconazole tablets has been approved

 简体中文
简体中文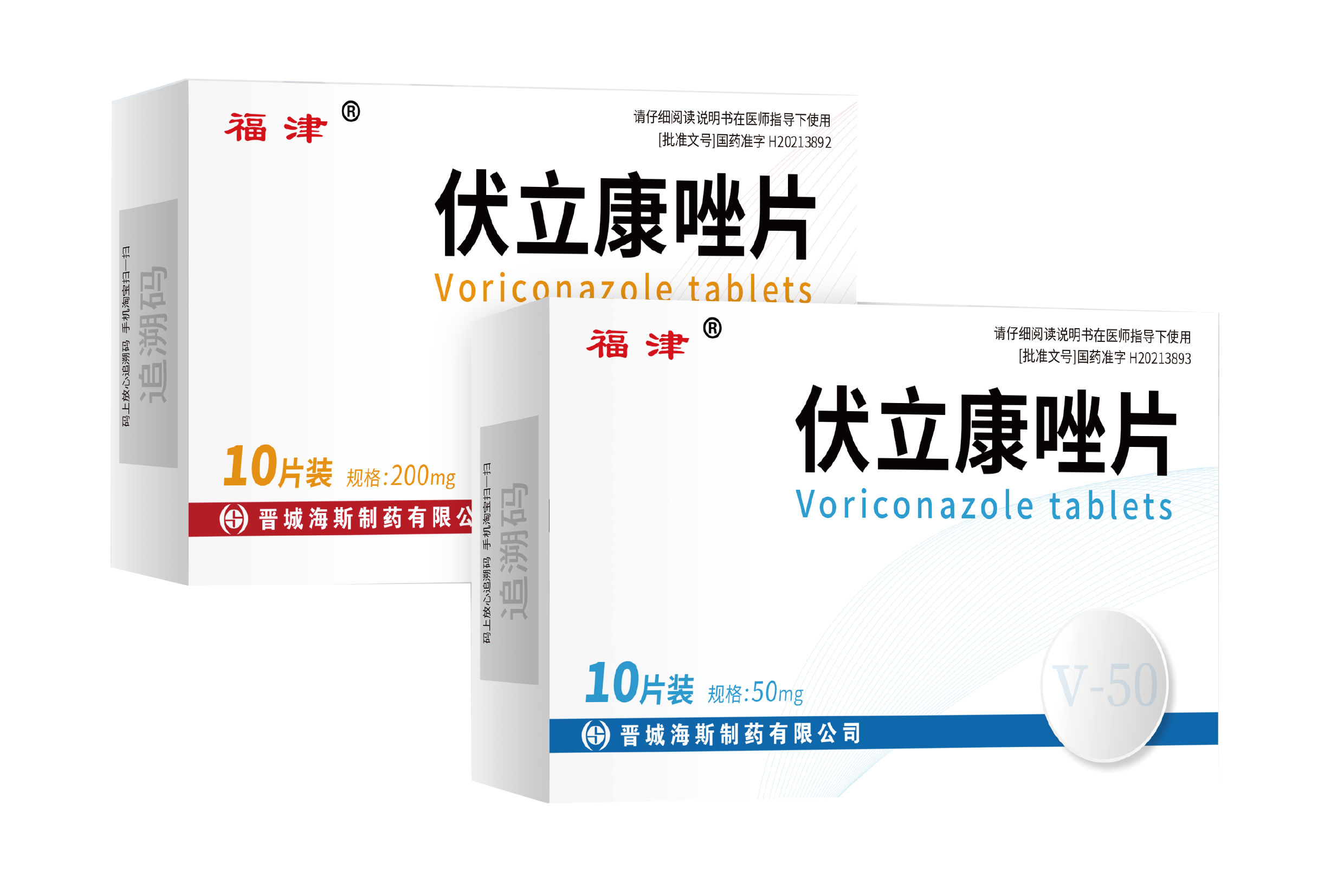

 86-10-67887855
86-10-67887855 Head Office:
Head Office: Marketing:
Marketing: Wista Pharmam
Wista Pharmam